Abstract
Background: The randomized CCTG LY.12 trial demonstrated that gemcitabine, cisplatin and dexamethasone (GDP) was non-inferior to dexamethasone, cytarabine, cisplatin (DHAP), with or without rituximab, in patients with relapsed or refractory (R/R) aggressive non-Hodgkin lymphoma (NHL) prior to autologous stem cell transplantation (ASCT) [ Crump JCO 2014 ]. A cost-utility analysis also proved GDP to be associated with both lower direct costs and similar quality-adjusted outcomes, and as such, is the preferred salvage regimen in this patient population [ Cheung JNCI 2015 ]. We conducted an analysis of such costs (lost productivity, paid and unpaid caregiver time) based on the trial data.
Methods: Resource utilization data were collected for all Canadian patients in the trial. Lost productivity data for both patients and caregivers were collected at baseline and with each chemotherapy cycle pre-ASCT, from a proportion of the Canadian patients, using an adapted Lost Productivity questionnaire. The human capital approach was utilized to calculate lost productivity costs using published Canadian salary standards according to field of employment [ http://www.statcan.gc.ca ]. Costs were presented in 2012 Canadian dollars from a societal perspective, and disaggregated to highlight the costs of patient lost productivity, paid caregiving hours and lost productivity from family members providing unpaid caregiving, and these were compared between the two treatment strategies. All statistical tests were two-sided, using the Wilcox rank sum test.
Results: Of the 519 Canadian pts in the trial, 374 completed at least 2 lost productivity instruments (72%) and are included in this analysis. In this subset, 62% of patients were male, the mean age was 53 years (range: 23-70 years), with 27% of patients being older than 60 years of age. There were no significant differences between the two arms with respect to demographics and lymphoma disease characteristics. As well, the lost productivity subset of patients was representative of the whole Canadian cohort, with no significant or meaningful differences in baseline characteristics.
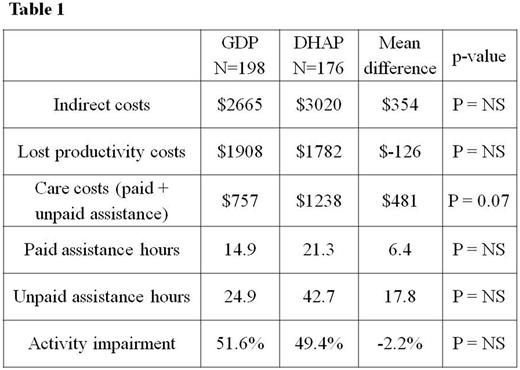
Average per patient indirect costs over the period of 2-3 cycles of chemotherapy were $2655 (95% CI $2253 to $3058) for patients in the GDP arm, an outpatient chemotherapy regimen, and $3010 (95% CI $2334 to $3686) for patients in the DHAP arm, a 3-day inpatient chemotherapy regimen (Table 1). There was no statistically significant difference in costs detected (difference $354; p=0.38). At salvage chemotherapy start, 69% of pts in the GDP arm and 73% in the DHAP arm were non-workers (on disability or sick leave). Of those still working full time at baseline, 3.5% reported decreasing their workload (part-time or stopped working) in the GDP arm, compared with 7.4% in the DHAP arm. Patients in both treatment arms reported substantial limitations in their usual activities, with approximately 50% impairment due to their health status.
The different cost components were disaggregated. Lost productivity of patients accounts for 65% of total indirect costs. Average patient lost productivity costs were $1908 with GDP and $1782 with DHAP (p = NS). Average care costs, including both paid and unpaid assistance, were $757 (95% CI $476 to $1037) with GDP vs. $1238 (95% CI $626 to 1850) with DHAP (difference $481; p=0.07). Over the period of 2-3 cycles of salvage chemotherapy, patients in the GDP arm paid for an average of 14.9 hours of care compared with 21.3 hours in the DHAP arm (p = NS) .
Thirty-nine pts (10.4%) did not have any unpaid caregivers, while the rest were cared for by a combination of: a spouse (68.5%), child/parent (34.8%), other relative (19.5%), friend (16.3%) or neighbor (2.9%). The average number of unpaid caregiver hours appeared lower with GDP (24.9 hrs; 95% CI 14.2 to 35.5) compared to DHAP (42.7 hrs; 95% CI 15.1 to 70.3).
Conclusions: Salvage chemotherapy for R/R aggressive NHL is associated with significant indirect costs to the patients and their caregivers, with a significant majority (approximately 70% of patients) not working or having to reduce their workload during this treatment time. Although there were no significant differences in all indirect costs between the two treatment arms, the paid and unpaid caregiving costs did appear higher with the DHAP arm. These data potentially provide further support for GDP being the preferred treatment approach in this patient population.
Prica: Celgene: Honoraria; Janssen: Honoraria. Hay: Janssen: Research Funding; Roche: Research Funding; Abbvie: Research Funding; Celgene: Research Funding; Novartis: Research Funding; Kite: Research Funding; Amgen: Research Funding. Crump: Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen Ortho: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.